Categories
New Blog
Tags
Are you struggling with inconsistent capsule filling accuracy in your pharmaceutical production? Capsule filling machines represent critical equipment in pharmaceutical manufacturing, yet achieving and maintaining optimal filling accuracy remains one of the industry's most persistent challenges.
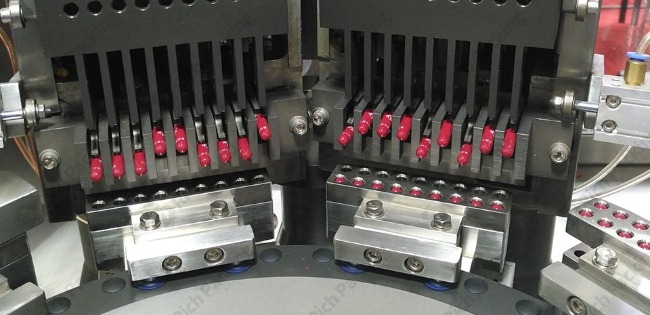
Capsule filling accuracy refers to the precision with which a capsule filling machine dispenses the exact target weight of pharmaceutical formulation into each capsule. This accuracy is typically measured as a percentage deviation from the target weight or as the relative standard deviation (RSD) across a batch. Industry standards generally require capsule weight variations to remain within ±3-5% of the target weight, though this may vary depending on regulatory requirements and specific product characteristics.
The importance of filling accuracy cannot be overstated in pharmaceutical manufacturing. Inaccurate filling can lead to significant consequences, including:
According to recent industry data, filling accuracy issues account for approximately 15-20% of all quality-related production delays in pharmaceutical manufacturing facilities. These challenges translate to significant financial implications, with estimates suggesting that filling accuracy problems cost the global pharmaceutical industry over $2 billion annually in waste, rework, and lost production time.

| Production Speed | Typical Accuracy (RSD) | Best Applications | Initial Investment | |
|---|---|---|---|---|
| Manual Filling | 100-300 capsules/hour | 3-8% | R&D, Very small batches | $500-$2,000 |
| Semi-Automatic | 3,000-15,000 capsules/hour | 2-5% | Small to medium production | $10,000-$50,000 |
| Automatic Intermittent | 25,000-90,000 capsules/hour | 1-3% | Medium to large production | $100,000-$350,000 |
| Continuous Motion | 80,000-200,000+ capsules/hour | 0.8-2% | High-volume production |
Dosator systems utilize a tubular dosing chamber with a piston that draws powder into the chamber through vacuum and then ejects it into the capsule body. These systems typically achieve a relative standard deviation (RSD) of 1-3% under optimal conditions.
The primary advantages of dosator systems include their versatility across a wide range of powder properties and their ability to handle moderately cohesive powders. However, they may struggle with extremely cohesive or very free-flowing materials, and their accuracy can deteriorate with wear of mechanical components.
Recent advancements in dosator technology include precision-engineered dosing chambers with specialized surface finishes that minimize powder adhesion and reduce variability. These innovations have improved filling accuracy by approximately 15-20% compared to older dosator designs.
Tamping finger systems use a series of compression steps to form a powder plug before transferring it into the capsule body. This technology typically achieves an RSD of 1.5-3.5% for most formulations.
These systems excel with difficult-to-flow powders and materials that require compression to achieve accurate dosing. The primary disadvantage is their complexity, with multiple mechanical operations increasing the potential for mechanical variation and maintenance requirements.
Modern tamping finger machines incorporate electronic monitoring of tamping force, allowing real-time adjustments to maintain consistent density and weight of the powder plug. This capability has reduced standard deviations by up to 30% compared to systems without force monitoring.
These systems use auger screws to transport powder coupled with vacuum assistance for precise metering. They typically achieve RSDs of 0.8-2% for suitable formulations, making them among the most accurate systems available.
Vacuum/auger systems offer exceptional accuracy for free-flowing granular materials and are particularly well-suited for low-dose applications. Their limitations include reduced effectiveness with cohesive or sticky formulations and more complex cleaning and validation requirements.
The latest generation of vacuum/auger systems incorporates microprocessor-controlled servo motors that provide precise control over auger rotation speed and duration, improving dose accuracy by up to 25% compared to conventional systems.
Powder flow properties significantly impact filling accuracy, with poorly flowing powders typically resulting in higher weight variations. The Hausner ratio and Carr's index serve as valuable indicators of how formulations will perform during the filling process.
| Flow Property Metric | Value Range | Flow Characteristic | Expected RSD Impact |
|---|---|---|---|
| Carr's Index | <10% | Excellent flow | Minimal (+0-0.5%) |
| Carr's Index | 11-15% | Good flow | Low (+0.5-1%) |
| Carr's Index | 16-20% | Fair flow | Moderate (+1-2%) |
| Carr's Index | 21-25% | Passable flow | Significant (+2-3%) |
| Carr's Index | 26-31% | Poor flow | High (+3-5%) |
| Carr's Index | 32-37% | Very poor flow | Very high (+5-8%) |
| Carr's Index | >38% | Extremely poor flow | Severe (+8%+) |
Research indicates that improving flow properties through formulation optimization can enhance filling accuracy by 30-50% for problematic materials. Common approaches include the addition of glidants like colloidal silicon dioxide or optimizing particle size distribution.
Uniform particle size distributions typically result in more consistent filling. Formulations with a narrow particle size range (represented by a lower span value) demonstrate improved flow characteristics and less segregation tendency, both contributing to better filling accuracy.
Studies have shown that formulations with a span value under 2.0 typically achieve 15-25% better filling accuracy compared to those with span values above 3.0. This highlights the importance of controlling particle size distribution during formulation development.
Micronized materials (particle size <10 μm) often present significant challenges for accurate filling due to their cohesive nature and poor flow properties. For such materials, specialized filling systems or formulation strategies may be necessary to achieve acceptable accuracy.
Moisture content significantly impacts powder behavior during the filling process. Optimal moisture levels typically range from 1-3% for most pharmaceutical formulations, with deviations beyond this range often leading to accuracy issues.
Excessive moisture (typically >4% for most formulations) can cause powder adhesion to machine surfaces, reducing flow and leading to underfilling. Conversely, excessively dry formulations (typically <0.5%) may generate static charges that disrupt normal flow behavior.
Environmental monitoring and control systems capable of maintaining relative humidity within ±5% have demonstrated improvements in filling accuracy of up to 20% compared to operations in uncontrolled environments. This highlights the critical importance of environmental control in achieving consistent filling performance.

Regular calibration directly correlates with filling accuracy performance. Evidence suggests that machines calibrated weekly show approximately 30% less deviation compared to those calibrated monthly or less frequently.
| Recommended Frequency | Impact on Accuracy | Common Verification Method | |
|---|---|---|---|
| Weight verification | Every 4-8 hours | Critical | Statistical sampling |
| Dosator/tamping pin inspection | Daily | High | Visual inspection |
| Component cleaning | Between batches/daily | High | Visual/swab testing |
| Full system calibration | Weekly | Very high | Standard weight testing |
| Mechanical wear assessment | Monthly | Medium | Dimensional verification |
| Comprehensive overhaul | Annually | Very high | OEM verification protocol |
Comprehensive preventive maintenance programs that include systematic component inspection and replacement of wear parts typically reduce filling accuracy deviations by 25-40% compared to reactive maintenance approaches. The most critical wear components affecting accuracy include dosing discs, tamping pins, and dosator seals.
Digital monitoring systems that track machine performance metrics over time can identify gradual accuracy drift before it exceeds specification limits. Such systems have been shown to reduce out-of-specification incidents by up to 50% through early intervention.
Operating speeds significantly impact filling accuracy, with most machines showing an optimal operational window. Data indicates that operating at 70-85% of maximum rated speed typically yields the best accuracy profiles for most equipment.
Production rates above 90% of maximum rated capacity commonly result in 15-30% higher standard deviations compared to optimal operating speeds. This degradation occurs due to reduced dwell time in the filling zone and increased mechanical vibration.
Additionally, rapid speed changes during production runs can temporarily disrupt filling accuracy. Gradual ramping of production speeds (5-10% increments with stabilization periods) has been shown to maintain better accuracy profiles compared to abrupt speed adjustments.
Environmental conditions significantly impact filling accuracy through their effects on powder properties. Research demonstrates that maintaining temperature within ±2°C and relative humidity within ±5% throughout production can improve filling consistency by 15-25%.
Cold spots within manufacturing facilities can cause condensation on machine surfaces, leading to powder adhesion and bridging. Temperature mapping studies have identified this as a common cause of filling variability in facilities with inadequate HVAC systems.
Modern capsule filling suites increasingly incorporate independent environmental control systems capable of maintaining precise conditions regardless of external facility variations. Such systems typically represent a 5-8% addition to capital costs but can reduce accuracy-related batch failures by up to 40%.
Strategic excipient selection significantly influences filling performance. Pharmaceutical development studies indicate that optimized excipient combinations can improve filling accuracy by 25-40% for challenging active pharmaceutical ingredients (APIs).
| Excipient Type | Function | Impact on Filling Accuracy | Common Examples |
|---|---|---|---|
| Glidants | Improve flow | Very high (+) | Colloidal silicon dioxide, Talc |
| Lubricants | Reduce friction | High (+) | Magnesium stearate, Stearic acid |
| Diluents | Bulk modification | Medium-high (+/-) | Microcrystalline cellulose, Lactose |
| Disintegrants | Dissolution | Low-medium (-) | Croscarmellose sodium, Crospovidone |
| Binders | Particle adhesion | Medium-high (-) | Povidone, HPMC |
Incorporating optimized levels of glidants (typically 0.2-1.0%) has demonstrated some of the most significant impacts on filling accuracy. Research shows that colloidal silicon dioxide at concentrations of 0.5-0.8% typically provides optimal flow enhancement without negatively affecting other formulation properties.
The selection of appropriate diluents based on the properties of the active ingredient represents another critical strategy. Microcrystalline cellulose typically improves accuracy for cohesive materials, while spray-dried lactose often enhances performance with poorly flowing APIs.
Particle engineering techniques offer powerful tools for enhancing filling accuracy. Granulation processes have demonstrated improvements in RSDs of 30-60% compared to direct compression blends of the same formulation.
Spray-dried and spray-congealed intermediates typically exhibit more spherical morphologies and improved flow properties. Studies indicate that these techniques can reduce weight variation by 20-35% compared to conventional blending approaches.
Co-processed excipients specifically designed for capsule filling applications have gained significant traction in recent years. These materials combine multiple functional excipients in a single engineered particle, providing optimized flow, compressibility, and lubricating properties that can improve filling accuracy by 15-30%.
Identifying and controlling Critical Material Attributes (CMAs) represents a key quality-by-design approach to filling accuracy. Research indicates that monitoring and controlling as few as 3-5 key material properties can predict filling performance with 85-90% accuracy.
| Analytical Method | Target Range (Typical) | Impact on Filling | |
|---|---|---|---|
| Bulk density | USP <616> | Formulation-specific | Direct correlation |
| Tapped density | USP <616> | Formulation-specific | Flow prediction |
| Hausner ratio | Calculation | 1.0-1.25 | Flow prediction |
| Particle size distribution | Laser diffraction | Formulation-specific | Flow and segregation |
| Moisture content | Loss on drying | 1-3% | Flow and adhesion |
| Electrostatic charge | Faraday cup | <2 nC/g |
Establishing specification limits for these attributes based on filling performance studies can significantly reduce batch-to-batch variability. Companies implementing this approach have reported reductions in filling-related deviations of 40-60% compared to traditional quality control approaches.
Real-time monitoring of critical material attributes during production, although still emerging as a technology, shows promise for further enhancing accuracy. Near-infrared and Raman spectroscopy techniques can provide continuous monitoring of blend uniformity and moisture content, potentially enabling proactive adjustments before accuracy issues occur.
The latest generation of dosing systems incorporates advanced engineering features specifically designed to enhance accuracy. Ceramic dosing chambers with specialized surface treatments have demonstrated 15-25% improvements in RSDs compared to conventional stainless steel components.
Multi-stage tamping systems with force-feedback control allow for adaptive compression based on real-time density measurements. This technology has shown particular promise for materials with variable bulk density, reducing RSDs by 20-35% compared to fixed-parameter tamping systems.
Microelectromechanical systems (MEMS) are beginning to appear in high-precision capsule fillers designed for low-dose applications. These systems can achieve extraordinary accuracy (RSDs <0.5%) for doses as low as 100 μg, expanding the range of medications suitable for capsule delivery.

In-line weight verification systems capable of checking 100% of produced capsules represent a significant advancement in filling technology. These systems identify and reject out-of-specification capsules in real-time while providing feedback to adjust filling parameters automatically.
Near-infrared (NIR) and Raman spectroscopy technologies increasingly serve as process analytical technology (PAT) tools for continuous monitoring of blend uniformity and active content. Implementation of these systems has been shown to reduce content uniformity failures by 30-50% through early detection of segregation or blending issues.
Machine learning algorithms that analyze patterns in filling data can now predict accuracy trends before they exceed specification limits. Early adopters of these technologies report 40-60% reductions in batch rejections through proactive intervention based on predictive analytics.
Clean-in-place (CIP) and wash-in-place (WIP) technologies specific to capsule filling equipment have significantly reduced the variability associated with manual cleaning procedures. Facilities implementing these systems report 15-30% improvements in first-batch success rates following changeovers.
Standardized, automated changeover procedures guided by electronic workflows reduce the variability associated with manual adjustments. Data indicates that such systems can reduce changeover-related accuracy deviations by 25-40% compared to traditional manual procedures.
Quick-change components with standardized interfaces facilitate rapid transitions between products while maintaining precision alignment critical for accuracy. These designs have reduced changeover times by 30-50% while simultaneously improving first-batch accuracy performance.
Regulatory expectations for capsule filling accuracy continue to evolve, with increasing emphasis on process understanding and control rather than end-product testing alone. Modern regulatory frameworks emphasize the implementation of process analytical technology (PAT) and quality-by-design (QbD) principles.
The FDA's Process Validation guidance emphasizes three stages of validation that directly impact filling accuracy: process design, process qualification, and continued process verification. This lifecycle approach requires ongoing monitoring of filling accuracy rather than periodic revalidation exercises.
International regulatory harmonization efforts have established common standards for weight variation and content uniformity. The International Council for Harmonisation (ICH) guidelines, particularly ICH Q8, Q9, and Q10, provide frameworks for ensuring consistent capsule filling accuracy across global manufacturing networks.
Modern validation approaches emphasize multifactorial design of experiments (DoE) to identify the operating space within which acceptable filling accuracy can be maintained. Studies indicate that comprehensive DoE approaches can reduce validation failures by 30-50% compared to traditional one-factor-at-a-time methodologies.
| Typical Parameters Assessed | Sample Size | Acceptance Criteria | |
|---|---|---|---|
| Installation Qualification | Component verification, utility requirements | N/A | Meets design specifications |
| Operational Qualification | Speed range, dosing range, controls | 3 settings | Functions as designed |
| Performance Qualification | Weight variation, content uniformity | 3 batches minimum | Meets pre-established criteria |
| Process Validation | Critical process parameters, material attributes | 3+ batches | Process capability indices ≥1.33 |
| Cleaning Validation | Residue limits, visual inspection | 3+ changeover cycles |
Risk-based validation approaches that focus intensive testing on high-risk parameters while applying less rigorous requirements to low-risk aspects have gained regulatory acceptance. This approach typically reduces validation costs by 20-30% while maintaining or improving quality assurance.
Continued process verification programs that collect and analyze filling accuracy data throughout the product lifecycle have become regulatory expectations. Such programs enable early detection of process drift and facilitate continuous improvement of accuracy performance.
Comprehensive SOPs covering all aspects of machine setup, operation, and maintenance form the foundation of accuracy control. Research indicates that facilities with detailed, updated SOPs demonstrate 25-40% fewer accuracy-related deviations compared to those with minimal documentation.
SOPs should include specific troubleshooting guidelines addressing common accuracy issues. Decision trees and visual aids within these documents have proven particularly effective, reducing resolution time for accuracy problems by 30-50% in many facilities.
Operator training programs tied directly to SOP compliance have demonstrated significant impacts on filling accuracy. Structured certification programs requiring demonstrated competency have reduced operator-related accuracy variations by 20-35% compared to informal training approaches.
Statistical sampling plans must balance efficiency with detection capability. Most pharmaceutical operations have adopted ANSI/ASQ Z1.4 sampling plans (formerly MIL-STD-105E) with acceptable quality limits (AQLs) of 0.65-1.0% for critical capsule weight parameters.
| Inspection Level | Batch Size | Sample Size | Application |
|---|---|---|---|
| S-2 | Any | Small | Quick process checks |
| I | <10,000 units | Reduced | Validated processes |
| II | Any | Normal | Standard monitoring |
| III | Any | Tightened | Following failures |
Automated weight sampling systems that integrate with production equipment have reduced the labor requirements for quality verification while increasing sampling frequency. These systems typically allow for 3-5 times more frequent monitoring without additional personnel costs.
Process capability indices (Cpk) serve as valuable predictive metrics for filling accuracy. Operations maintaining Cpk values above 1.33 for filling weight demonstrate 60-80% fewer batch failures compared to those operating with marginal capability (Cpk < 1.0).
Systematic root cause analysis of accuracy deviations yields more effective corrective actions than empirical adjustments. Facilities employing structured problem-solving methodologies report 40-60% higher success rates in permanently resolving recurring accuracy issues.
| Common Causes | Diagnostic Approach | Typical Resolution | |
|---|---|---|---|
| Consistent underfilling | Insufficient powder in feed tray, Mechanical wear | Check feed system, Inspect dosing components | Adjust feed system, Replace worn components |
| Consistent overfilling | Incorrect machine settings, Compression issues | Verify settings, Check tamping pressure | Reset parameters, Adjust compression |
| High variability | Flow problems, Environmental fluctuations | Analyze flow properties, Check environment | Formulation adjustment, Control environment |
| Trending weight change | Powder settling, Component wear | Check powder level, Inspect for wear patterns | Modify powder management, Replace components |
| Intermittent failures | Vibration, Electrical interference | Monitor vibration, Check electrical systems |
The development of failure mode and effects analysis (FMEA) specific to filling operations has proven valuable for proactive accuracy management. Operations implementing FMEA-based preventive actions report 30-45% reductions in unplanned accuracy-related interventions.
Achieving consistent capsule filling accuracy requires a comprehensive understanding of the complex interplay between machine parameters, material properties, and environmental factors. By implementing the strategies outlined in this article, you can significantly enhance your operation's filling precision and product quality.
Would you like to discuss your specific capsule filling challenges? Contact our expert team today for a personalized consultation to optimize your capsule filling accuracy and efficiency.