Categories
New Blog
Tags
What critical factors affect the production quality of the capsule filling machine, influencing everything from dosage accuracy to regulatory compliance? Understanding these elements, spanning machine capabilities, material properties, and operational procedures, is paramount for achieving consistent, high-quality pharmaceutical or nutraceutical capsule production. Let’s delve into the details.

In the pharmaceutical and nutraceutical industries, the quality of the final capsule product is non-negotiable. It directly impacts patient safety, therapeutic efficacy, and brand reputation. Substandard capsules, resulting from poor control over the filling process, can lead to incorrect dosages, stability issues, patient non-compliance, and costly product recalls. Regulatory bodies like the FDA and EMA enforce strict Good Manufacturing Practices (GMP) that mandate precise control over every manufacturing step, including capsule filling. Therefore, mastering the factors that affect the production quality of the capsule filling machine is essential for compliance and market success.

Before dissecting the influencing factors, let's briefly outline the typical stages involved in an automatic or semi automatic capsule filling machine operation:
Each stage presents potential challenges where quality can be compromised if specific factors are not adequately controlled.
Achieving consistent, high-quality capsule production requires a holistic view. The factors that affect the production quality of the capsule filling machine can be broadly categorized. We will explore each in detail. These include the machine itself, the empty capsules used, the formulation being filled, operational parameters, environmental conditions, and maintenance practices. Each plays a vital role in the final product outcome.
The inherent design, engineering precision, and build quality of your capsule filling machine form the foundation for production quality. Inferior design or construction inevitably leads to inconsistencies.
The level of automation significantly impacts potential quality variations.
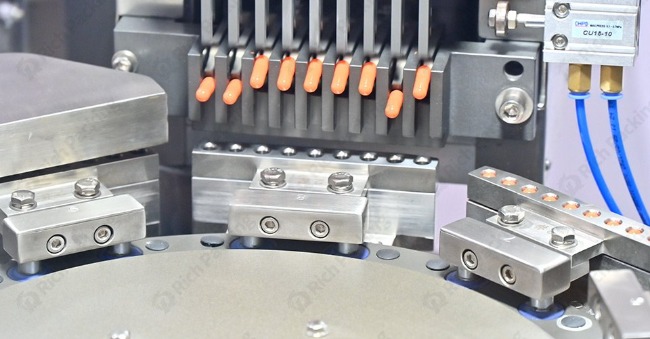
High-quality machines are built with tight tolerances. This applies to the movement of the turret, the alignment of capsule segments or bores, the precision of dosing mechanisms, and the fit of tooling (like dosing discs, tamping pins, and capsule holding segments). Poor tolerances lead to inconsistent capsule handling, variable fill weights, and potential capsule damage.
Contact parts are typically required to be made of pharmaceutical-grade stainless steel (e.g., 316L SS) or other approved non-reactive materials. This prevents contamination, ensures cleanability, and resists corrosion from various formulations. Using appropriate materials is a fundamental factor that affects the production quality of the capsule filling machine by ensuring product purity.
The heart of the machine is its dosing system. Common types include:
The mechanisms responsible for orienting, separating, closing, and ejecting capsules must operate smoothly and precisely. Misaligned or poorly designed separation stations can damage capsule rims. Ineffective closing stations can result in improperly locked or dented capsules ("telescoping"). Gentle ejection prevents damage to finished capsules. These mechanical actions are vital factors that affect the production quality of the capsule filling machine.
Modern automatic capsule filling machine units often incorporate sensors to detect:
A robust Programmable Logic Controller (PLC) ensures repeatable and precise control over machine timings, speeds, pressures, and sequences. An intuitive Human-Machine Interface (HMI) allows operators to accurately set parameters, monitor the process, manage alarms, and access batch data. Reliable controls are essential for consistent operation, a major factor that affects the production quality of the capsule filling machine.
The quality and consistency of the empty capsules themselves are critically important. Even the best capsule filling machine cannot compensate for poor-quality capsules.
Empty capsules must adhere to strict dimensional specifications: overall closed length, cap length, body length, wall thickness, and diameters (cap and body). Variations can cause issues with:
Capsules must be strong enough to withstand the mechanical stresses of the filling process (separation, filling, closing, ejection) without cracking, splitting, or denting. Brittleness (often due to low moisture content) or excessive softness (high moisture) can lead to defects. Ensure proper storage conditions.
Different capsule materials (e.g., traditional gelatin, HPMC/Vcaps®) have slightly different properties regarding moisture sensitivity, brittleness, and static charge accumulation. The capsule filling machine settings might need minor adjustments (e.g., pre-separation vacuum, closing pressure) depending on the capsule type being used. Ensure compatibility between the machine setup and capsule material.
The surface smoothness and any residual manufacturing lubricants on the capsules can affect how they feed and interact with machine parts. Excessive static charge can also cause handling problems. These subtle properties are contributing factors that affect the production quality of the capsule filling machine.
The physical characteristics of the powder, granules, or pellets being filled significantly impact the filling process and final product quality. Formulation properties are often the most challenging factors that affect the production quality of the capsule filling machine.
This is arguably the most critical powder property. Poor powder flow results in:
A powder with a wide PSD or excessive fines can lead to segregation issues within the hopper and dosing system, contributing to weight variation. Fines can also cause dusting issues and potentially affect tooling lubrication or movement if excessive. Consistent PSD is crucial.
These properties influence how much powder mass occupies a given volume. Variations in density directly impact fill weight accuracy, especially in volumetric dosing systems (like tamping pin/dosing disc). Consistent density throughout the batch is vital. Ensure proper blending techniques.
For tamping pin systems, the powder must form a stable, handleable slug under compression without sticking to the pins or crumbling upon ejection into the capsule body. Poor compressibility leads to weight variation and potential loss of formulation during transfer.
Formulations that readily absorb moisture from the environment can exhibit changes in flowability, density, and compressibility during a production run, leading to inconsistencies. Environmental control (humidity) becomes critical for such products. This interaction is a key factor that affects the production quality of the capsule filling machine.
Powders prone to static electricity can cling to machine parts (hopper walls, tooling), hindering flow and causing inaccurate dosing. Proper grounding of the capsule filling machine and sometimes humidity control or anti-static additives in the formulation can mitigate this.
Ensuring the Active Pharmaceutical Ingredient (API) is uniformly distributed throughout the excipient blend is critical for content uniformity in the final capsules. This relies on proper blending processes before the powder reaches the capsule filler. While not a machine factor per se, inconsistent input material directly impacts output quality.
How the capsule filling machine is set up and operated by trained personnel directly influences the quality of the output.
Each product may require specific, validated settings for:
Well-trained operators are crucial, especially for setup, monitoring, adjustments, and troubleshooting. They need to understand:
Regular checks during the production run are essential to confirm quality is maintained. Common IPQCs include:
Clear, detailed, and validated SOPs for machine setup, operation, cleaning, and changeover are essential for ensuring consistency across different batches and operators. Adherence to SOPs minimizes variability.
The manufacturing environment can significantly influence both the formulation and the machine's performance.
As mentioned, temperature and humidity affect:
The filling suite must be maintained in a clean state according to GMP standards. Dust or residue from previous batches can contaminate the product. Proper room cleaning procedures and air handling systems (HVAC with appropriate filtration) are necessary.
Excessive vibration from nearby equipment or the building structure can potentially affect the precision of sensitive machine parts and dosing mechanisms, especially at higher speeds. Isolating the capsule filling machine or addressing the vibration source may be necessary.
Proper maintenance ensures the capsule filling machine continues to operate reliably and accurately over time. Neglect leads to deteriorating quality.
Adhering to a manufacturer-recommended preventative maintenance schedule is crucial. This includes:
Capsule-holding segments, tamping pins, dosing discs, and dosator nozzles are precision components. They must be handled carefully, cleaned thoroughly according to validated procedures, inspected regularly for wear or damage (nicks, scratches, bending), and stored properly. Damaged tooling is a direct cause of capsule defects and fill weight issues. Tooling condition is one of the most direct factors that affect the production quality of the capsule filling machine.
Thorough cleaning between batches is essential to prevent cross-contamination. Cleaning procedures must be validated to demonstrate effectiveness in removing product residues. Inadequate cleaning compromises product purity and can also impede machine function if residues build up on moving parts.
| Specific Elements | Primary Quality Impact | Mitigation Strategy | |
|---|---|---|---|
| Machine Design | Precision, Materials, Dosing System, Handling, Controls | Fill Weight Accuracy, Capsule Defects, Consistency | Invest in Quality Machine, Proper Specification |
| Machine Type | Automatic vs. Semi Automatic Capsule Filling Machine | Consistency, Operator Influence, Speed vs. Flexibility | Choose Type Based on Scale & Needs, Operator Training |
| Empty Capsules | Dimensions, Strength, Material, Storage | Handling Issues, Separation/Closing Defects, Breakage | Reputable Supplier, Proper Storage, QC Checks |
| Formulation | Flowability, PSD, Density, Compressibility, Static | Fill Weight Variation, Content Uniformity, Dusting, Handling | Formulation Development, Excipient Selection, Granulation |
| Operational | Setup Parameters, Speed, Operator Skill, IPQCs, SOPs | Fill Weight Accuracy, Defects, Consistency, Compliance | Validation, Training, Strict Procedures, Monitoring |
| Environmental | Temp, Humidity, Cleanliness, Vibration | Capsule/Powder Integrity, Contamination, Machine Stability | HVAC Control, GMP Cleaning, Facility Design |
| Maintenance/Cleaning | PM Schedule, Tooling Care, Validated Cleaning | Machine Reliability, Accuracy, Defects, Cross-Contamination |
Mastering the multitude of factors that affect the production quality of the capsule filling machine is essential for any pharmaceutical or nutraceutical manufacturer. It requires a synergistic approach combining well-designed equipment like a reliable automatic capsule filling machine or a well-operated semi automatic capsule filling machine, high-quality input materials (capsules and formulation), tightly controlled operational parameters, a suitable environment, and diligent maintenance. Addressing each factor systematically leads to consistent, compliant, and high-quality capsule products.
Do you have questions about optimizing your capsule filling process or selecting the right equipment? Contact our experts today for personalized advice and solutions tailored to your specific production needs.